Close
Shop All:Innovating Science
Innovating Science AP Chemistry Determination Of Water Hardness
Item #:
2134237
Sign In
To See Your Price
package_2 Free Shipping Eligible.
Quickship Eligible.
Ships from Manufacturer Typically Within 2-4 Weeks - Lead Times Vary
In this experiment the indicator eriochrome black T (EBT) is used to signal the presence of ions in the water sample.
About This Item
Description
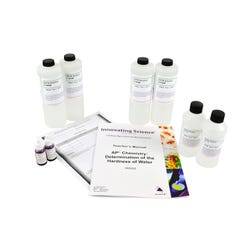
EBT binds with free metal ions in the water to form a pink complex. EDTA has a stronger affinity for the metal ions than EBT so when EDTA is added it replaces the EBT and the EBT returns to its blue, uncomplexed color. The blue color is used as the end point in the titration. A sample of tap water is treated with EBT indicator. If the indicator turns from blue to pink, metal ions such as calcium and magnesium are present. To determine the concentration of ions present, the sample is titrated with a known molar concentration of EDTA.Features
Includes:
- 4 X 500 mL EDTA solution, 0.05 M
- 2 x 200 mL buffer solution
- 2 x 15 mL EBT Solution (Erichrome Black, 0.1%)
Shipping Type:
parcel
Free Shipping:
true
Attachments
Specifications
UPC:
724832080206
Maximum Age:
18+
Maximum Grade Level:
HS+